Mobile Room UVC Germicidal Solutions for Healthcare
ARTZ 2.0® Mobile Room UVC Germicidal Solutions for Healthcare
Automatically, Reliably Targeting Zero (ARTZ) microorganisms, using the proven power of UVC disinfection in the healthcare environment, is the goal of the ARTZ® 2.0 mobile room solution unit. The ARTZ 2.0 is a re-design of the original ARTZ 1.0, but now has faster disinfection cycles, a simplified user interphase, and the necessary document control features desired by our healthcare clients.
This customer favorite is used as adjunct cleaning technology from small patient care environments (treatment rooms and offices); Emergency Rooms; patient rooms after terminal clean; hospital bathrooms; hospital cafeterias and common areas; children's hospitals for enhanced play area disinfection, to large Operating Room Suites. The ARTZ 2.0 is found anywhere adjunct UVC disinfection is needed. The ARTZ 2.0 is non-intimidating and can be quickly learned by any user. It is less expensive, more efficient, and more effective than any other comparable mobile room germicidal UVC disinfection device in the marketplace.
Here's what makes the ARTZ 2.0® so unique:
Affordable
- Economically priced high-end system; Inquire today to learn more.
- Individually validated lamps rated for 12,000 hours, which exceeds all other mobile room UVC devices
Fast
- Sets up quickly with any web-enabled device of your choosing (mobile phone, tablet, or laptop)
- Features high-output UVC flux
- Disinfects high touch non-critical surfaces from microorganisms in minutes
Effective
- Delivers balanced and uniform UVC flux to surfaces and air; automatically delivers UVC dose needed to reduce microorganisms that include:
- Methicillin resistant Staphylococcus aureus (MRSA)
- Clostridioides difficile – spore forming bacteria
- Acinetobacter baumannnii
- Vancomycin resistant enterococcus (VRE)
- Extended Spectrum Beta Lactamase Producers (ESBL) – gram negative bacteria
- Influenza virus (Avian, Influenza A)
- Other bacteria, virus, or fungi (please contact American Ultraviolet regarding microbes of interest not listed)
- Preset dosage recipes are built into the system to target desired microbes; if preferred, ARTZ 2.0® can be set manually to provide required dose
Safe
- 360° motion sensors - ARTZ 2.0® automatically shuts off if room is entered for any reason during treatment
Downloadable Data
- Automatic document control data logging helps reduce or eliminate human error during data collection
- Wirelessly downloads acquired session data to a computer
- Measures productivity and time management
- The ARTZ 2.0® records session log-in, users, dates, device locations; device run time; device settings; reporting
User-friendly
The ARTZ 2.0® can be controlled from the web-enabled device of your choosing (mobile phone, tablet or laptop) - so no need to worry about a lost, damaged, or stolen controller. Simply connect to the ARTZ 2.0's discrete Wi-Fi network and log in to web-based controls that offer cycle logging, fault logging, and lamp status information.
- Easy-to-operate; Minimal training required
- Adjunct to standard terminal cleaning process
- Controlled by any web-enabled device of your choosing (mobile phone, tablet, or laptop)
- 360° handle for ease of transport
- Multiple languages available
Green Technology
- Produces no ozone or other secondary contaminants
- No waste is generated
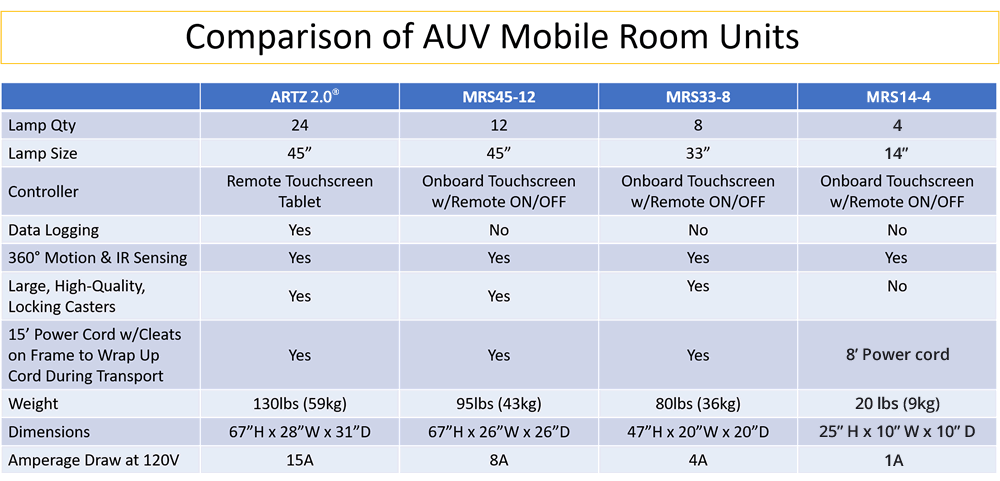
MRS45-12 Mobile UV Unit
The MRS45-12 mobile UVC device is ideal when very fast UVC disinfection times are needed for small, medium, or large areas, including patient care environments, operating suites, nursing homes, emergency shelters, large exercise facilities, gymnasiums, meeting rooms, manufacturing facilities, and prisons / jail cells. The MRS45-12 is designed to accommodate all environmental service applications, and due to its user-friendly design, can be found "in all spaces."
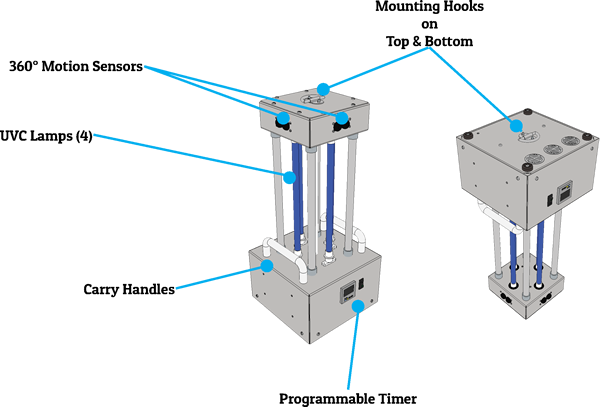
This unit uses 360-degree motion sensors as a safety precaution and features twelve (12), 45" slimline UVC lamps for maximum intensity. The unit is controlled via a WiFi-enabled device (phone/tablet) with 3 pre-programmed disinfection cycles, and the option to manually set disinfection cycle times.

Features
- Control with WiFi-enabled device (phone/tablet)
- Track user and location
- Downloadable cycle reports
- 304 stainless steel construction
- 360° motion sensors for automatic shut-off
- Large, high quality, locking casters for ease of transport
- 120/220 Volt, 50/60 Hertz
- Formed stainless tube structure protects lamps and improves ability to handle and transport the unit
- Access panel for ease of maintenance
- 15-foot power cord
Technical Specifications
MRS33-8 Mobile UV Unit
The MRS33-8 mobile UVC device can quickly disinfect medium to large-sized rooms, including healthcare environments, patient rooms, restaurants, office spaces, elevators, daycare environments, nursing and long-term care facilities, schools and universities, emergency shelters, exercise rooms and gymnasiums, spas, bathrooms, and shower areas. It can also fit into public transportation vehicles for quick disinfection and will comfortably fit in the back of emergency medical vehicles.
This unit uses 360-degree motion sensors as a safety precaution and features eight (8), 33" slimline UVC lamps optically centered around a highly polished reflector for maximum intensity. The unit is controlled via a WiFi-enabled device (phone/tablet) with 3 pre-programmed disinfection cycles, and the option to manually set disinfection cycle times.

Features
- Control with WiFi-enabled device (phone/tablet)
- Track user and location
- Downloadable cycle reports
- 304 stainless steel construction
- Motion sensor for 360-degree automatic shut-off
- Large, high quality, locking casters for ease of transport
- 120/220 Volt, 50/60 Hertz
- Formed stainless tube structure protects lamps and improves ability to handle and transport the unit
- Access panel for ease of maintenance
- 15-foot power cord
Technical Specifications
MRS14-4 Mobile UV Unit
The MRS14-4 mobile UVC device has proven to be a favorite solution for Emergency Medical Services, firefighters, law enforcement vehicles, school environments, restaurants, workplace areas, bathrooms, veterinary offices / kennels, and really all environments with a smaller footprint. It can be hung from the top or bottom mounting hooks, placed on desks, and easily moved around on rolling carts. It quickly disinfects surfaces at shorter distances and is equipped with the important 360° safety features that help prevent accidental UVC exposure. For ease-of-use it is equipped with a remote control for quick start, and with its high portability, you can see why the MRS14-4 has become a real bestseller.
This unit uses 360-degree motion sensors as a safety precaution and features four (4), 14" slimline UVC lamps optically centered around a highly polished reflector for maximum intensity. System controls are located directly on the unit, which allows utilization of an electronic timer with user selectable disinfection cycle times, and the option to manually set disinfection cycles times.
 Features
Features
- 360° motion + IR sensor safety shut-off
- Type 304 stainless steel construction
- Remote control for cycle start/stop
- Eye hooks on top and bottom to suspend unit upright or upside down
- Programmable cycle timer
- Main power switch
Technical Specifications
- Weight: 20 lbs. (9 kg)
- Dimensions: 25"h * 10"w * 10"d (64cm * 25cm * 25cm)
- Lamps: qty (4) GML600A
- Electrical Requirements:
- 1A @ 120V
- Power Cord: 8ft (2.4m)
- Construction: 304 stainless steel
Companion Products
Germicidal Ultraviolet Expertise - 60+ Years of Experience
Our mobile room disinfection units, and the Blade handheld unit, are manufactured in the USA by American Ultraviolet. Since 1960 American Ultraviolet has manufactured more than 100,000 UVC germicidal fixtures that are safely operating in hospitals, laboratories, clean rooms, doctor's offices, commercial buildings, food processing plants and residences throughout the world – any place a concern for adjunct disinfection exists. American Ultraviolet is the leader in UV decontamination technology, and we remain committed to providing the highest quality UVC germicidal equipment at a fair price. We stand fully behind our line of mobile room disinfection units, and all of our ultraviolet light decontamination equipment. We offer full, direct factory support, installation, and training.
To learn more about mobile room disinfection units, the Blade handheld unit, and other hospital UV products, please visit our technical library where you can download and print literature.
Prolonged, direct exposure to UVC light can cause temporary skin redness and eye irritation. American Ultraviolet systems are designed with safety in mind and, when properly installed, do not allow exposure to UV radiation and allow for safe operation and maintenance.
The systems are designed to provide UV-C energy. No warranty or responsibility is taken by American Ultraviolet in regards to the process or efficacy of the unit as American Ultraviolet has no control over the use of the system. American Ultraviolet makes no claims to the actual log reduction of microorganisms as it can greatly vary depending on environment. UV-C is an adjunct cleaning method and dirt, dust, and debris need to be removed from surfaces before UV-C treatment.
None of the American Ultraviolet UVC products detailed above are certified, or approved under any applicable laws, as a medical device, and as such, American Ultraviolet, and its Representatives and Distributors, do not currently intend for them to be used as medical devices anywhere globally. Products have not been evaluated by the FDA.